Neuralink, founded by Elon Musk and a group of engineers in 2016, was already under the scanner of a federal investigation regarding its animal trial program and is yet again facing new allegations of malpractice from the animal rights advocacy group Physicians Committee for Responsible Medicine (PCRM).
The company is attempting to build a brain chip interface that could be implanted within the skull, which according to the company, will help disabled patients to move as well as communicate again and also restore vision – enabling the paralyzed to walk and the blind to see!
It is presently in the animal trial stage; however, it has raised concerns after the physician’s committee of responsible medicines in its animal welfare advocacy group wrote to the secretary of transportation in the U.S. and alerted the same on documents it obtained on the matter.
The records acquired contain emails and other related material that suggest unsafe packaging and movement of plants obtained from the brains of monkeys.

What Is The Concern?
The organization claims that the biotech company illegally and improperly transported hazardous materials potentially contaminated with dangerous primate pathogens.
PCRM on Thursday sent a letter outlining Neuralink’s alleged violations and appropriate supporting evidence to the Department of Transportation. This U.S. agency is responsible for regulating and overseeing the transportation of hazardous materials. In the letter, the animal-welfare group requested that the DOT should look into Neuralink’s activity and issue appropriate fines for violations.
It said that the company’s documented track record is sloppy and indulges in unsafe laboratory practices.
This Is A Serious Concern?
It seems that after DOT received the complaint from PCRM, it took the same seriously, and it plans to conduct an investigation to make sure that Neuralink is fully compliant with federal laws.
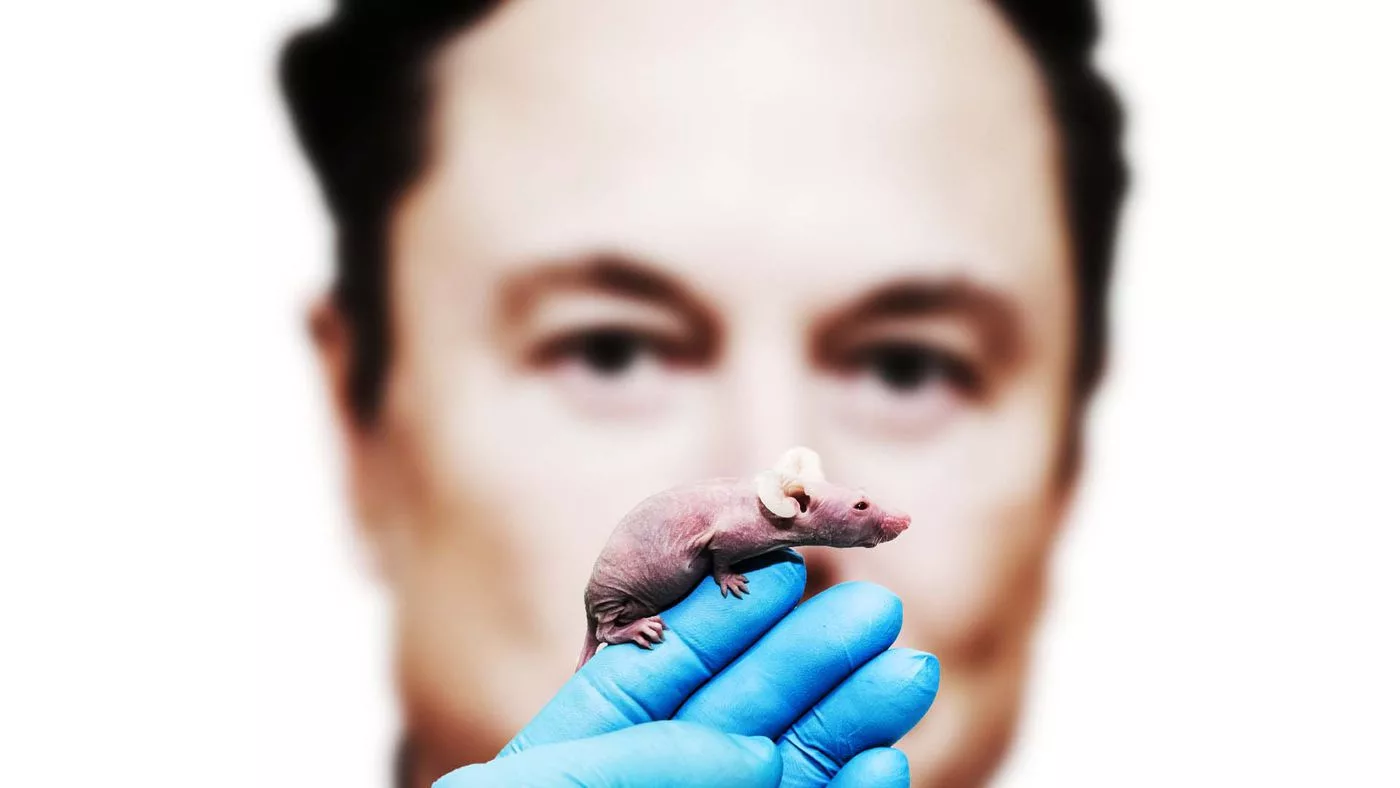
The dangerous materials in question are Neuralink devices implanted and later extracted from the brains of rhesus monkeys that may have been contaminated with multiple pathogens.
The complaint mainly focuses on “Animal 13”, a female macaque with two Neuralink implants inserted in its brain.
Following the insertion surgery, Animal 13 developed an apparent infection, while Neuralink researchers tried to treat Animal 13’s infection and then later killed the monkey—as is standard in animal testing trials.
The Neuralink device was removed, and a necropsy examination revealed that Animal 13 was infected with multiple pathogens, including an antibiotic-resistant form of staph and a type of bacterial pneumonia.
According to CDC, as a rhesus macaque, the monkey may have also been infected with herpes B, a virus for which the species is a natural reservoir that can cause severe brain damage and death in humans in the rare instance that transmission occurs.
What is worth noting is that emails exchanged between UC Davis and Neuralink personnel, and included in PCRM’s letter, seem to indicate that the unsterilized, contaminated device removed from Animal 13 in March 2019 was transported from UC Davis’ primate facility to a Neuralink facility by someone without the proper hazardous materials training.
Further, the emails suggest that the contaminated device was not appropriately packaged during transport or subsequent storage.
The documents provided by PCRM also account for at least two later cases of devices removed from infected monkeys, also from 2019.
In those instances, the devices were transported by adequately trained personnel, but they still may not have been packaged according to the relevant safety standards.
An email from an unidentified UC Davis employee describes all three devices as having “made their way back on site in an open box with no secondary container.”
This is an exposure to anyone coming in contact with the contaminated explanted hardware, and we are making a big deal about this because we are concerned for human safety.
The documents provided by PCRM don’t catalogue any resulting instances of human harm from Neuralink’s alleged negligence. But failing to manage hazardous materials properly is illegal in its own right, and violators can face civil penalties of about $100,000 per incident.
For context: To minimize the safety risk, extensive laws dictate how potentially infectious materials like medical waste and blood samples must be transported.
Hardware collected from inside the brains of disease-carrying monkeys falls under that umbrella of regulated, hazardous stuff. And an “open box” doesn’t generally cut it when it comes to storing something contaminated with an infectious pathogen.
The company is already facing scrutiny for alleged animal welfare violations. It is under USDA investigation for the abuse of monkeys and other animals used in testing,
Earlier in 2022, the advocacy group filed a lawsuit against UC Davis for its involvement in Neuralink experiments, alleging that the company’s animals were subject to horrific conditions amounting to torture.
Neuralink has previously denied animal welfare violations and said it is “absolutely committed to working with animals most humanely and ethically possible.”
Though Musk has set big goals for Neuralink, the company has yet to deliver on its lofty promises.
In a “show and tell” demonstration in December 2022, the CEO offered little news. In 2021, Neuralink released a video allegedly showing a monkey with a Neuralink implant playing a game of Pong “telepathically.”
However, though it might seem impressive, that demo is effectively just a repeat of an experiment from nearly 20 years ago. Neuralink has repeatedly missed its self-set deadlines for entering human trials.
What Progress Has The Company Made So Far?
Neuralink has delivered several examples of testing aspects of its technology successfully on animals, which also included a video in 2021 that showed a macaque playing a simple videogame after it had been implanted with a brain chip; in its presentation, it showed the improvements in the speed and capabilities of the chip.
Neuralink has yet to secure U.S. regulatory approval to move to human trials – unlike competitor Synchron, which has less ambitious goals for its medical advances.
Neuralink has missed Musk’s publicly stated deadlines to start human trials; Musk also said he thinks Neuralink can start human clinical trials in a timeframe of six months.