Zydus Cadila starts supply of COVID-19 vaccine to the government

Zydus Cadila starts supply of COVID-19 vaccine to government
Drug firm Zydus Cadila on Wednesday said it has commenced supplies of its COVID-19 vaccine ZyCoV-D to the central government.
The company has initiated the supply as per the order placed by the government, the drug firm said in a statement.
The group is also planning to make the vaccine available in the private market, it added.
ZyCoV-D is a three-dose vaccine administered intradermally.
“The vaccine will be priced at Rs 265 per dose and the applicator being offered at Rs 93 per dose, excluding GST,” Zydus Cadila said.
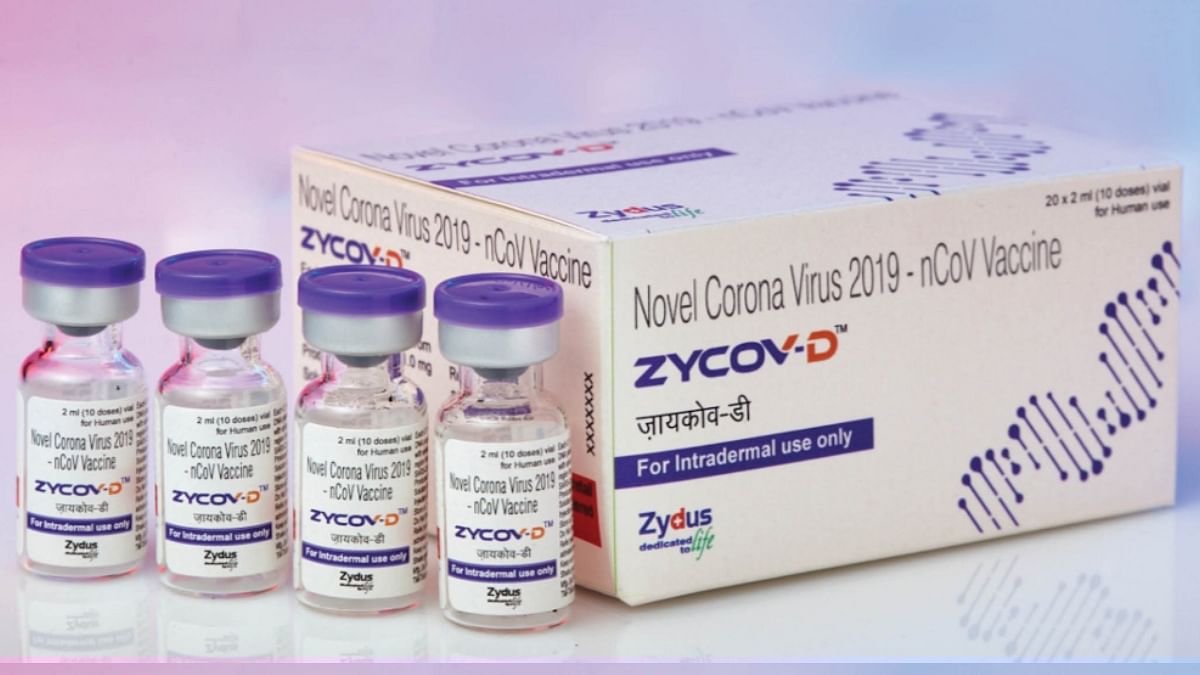
The Ahmedabad-based drugmaker has also entered into a definitive agreement with Shilpa Medicare Ltd, a contract manufacturing organisation, to produce mutually agreeable doses of ZyCoV-D.
It has also entered into an agreement with Enzychem Lifesciences of the Republic of Korea for the manufacturing license and technology transfer for the Plasmid DNA Vaccine, Zydus Cadila noted.

ZyCoV-D is a Plasmid DNA vaccine that produces the spike protein of the SARS-CoV-2 virus and elicits an immune response mediated by the cellular and humoral arms of the human immune system, which play a vital role in protection from disease as well as viral clearance.
Article Proofread & Published by Gauri Malhotra.



